Introduction
Our teeth are exposed to many environmental influences every day. Acids from food and drink attack our enamel in the same way as bacterial acids. Therefore, it is important that our teeth are well protected. Several studies have already shown that after using products with hydroxyapatite, these particles form a protective layer on our teeth. However, until now it was not scientifically evaluated how much of the surface is covered with these particles after single use of hydroxyapatite products.
Question
How much of the tooth surface is covered with hydroxyapatite after single use?
Material and methods
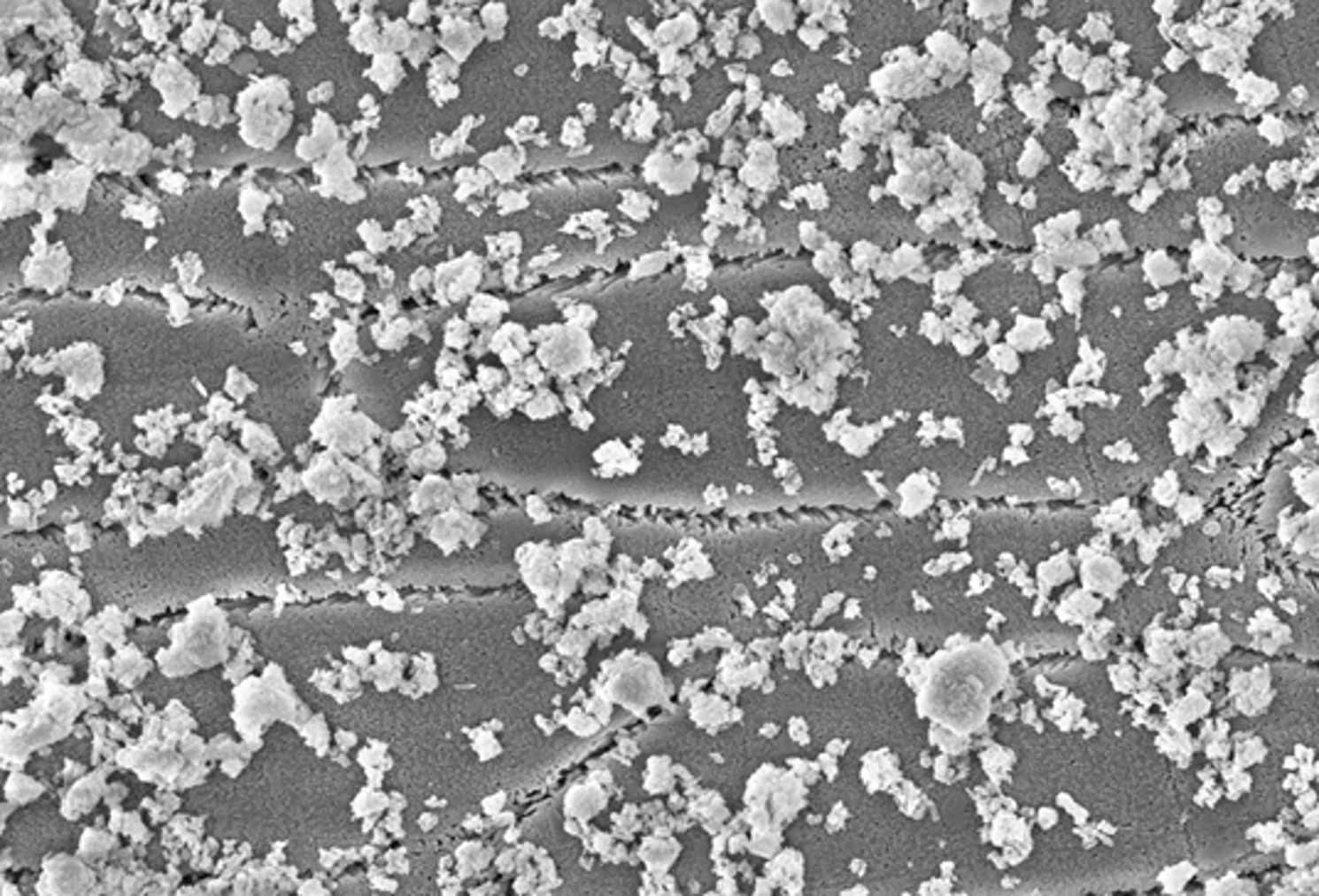
This in vitro study was conducted at the Max-Planck-Institute in Düsseldorf, Germany. Enamel slabs from bovine teeth were immersed in aqueous dispersions with hydroxyapatite particles. The following concentrations of hydroxyapatite particles were used: 1, 5 and 10%. The slabs were then examined using scanning electron microscopy. The degree of coverage of hydroxyapatite on the tooth surface was measured and quantified.
Results
The tooth samples were visibly covered after single immersion in an aqueous dispersion with microcrystalline hydroxyapatite particles. With a 10% hydroxyapatite dispersion, more than one third of the surface was covered with a layer of hydroxyapatite after just a short time. Furthermore, the microscopic images indicated that the particles had firmly adhered to the surface of the teeth.
Conclusion
After just one use of dental care products with hydroxyapatite, a protective layer forms on the surface of the teeth. This layer makes it difficult for bacteria and acids to attack the natural enamel.
For more information read the study titled "Quantitative affinity parameters of synthetic hydroxyapatite and enamel surfaces in vitro".
Source: Fabritius-Vilpoux, K., Enax, J., Herbig, M., Raabe, D. & Fabritius, H.-O. Quantitative Affinity Parameters of Synthetic Hydroxyapatite and Enamel Surfaces in vitro. Bioinspired, Biomimetic and Nanobiomaterials 8, 141-153 (2019).